PIPELINE
Bridge Biotherapeutics currently have two NCEs, BBT-401 and BBT-877 in development.
| Program | Indication | 2017 | 2018 | 2019 |
|---|---|---|---|---|
| BBT-401Pellino-1 Inhibitor | Ulcerative Colitis |  |
||
| BBT-877Autotaxin Inhibitor | Idiopathic Pulmonary Fibrosis |  |
||
| Next Generation Pellino Inhibitor |
Development Stage |  |
||
| New Project | Development Stage |  |
||

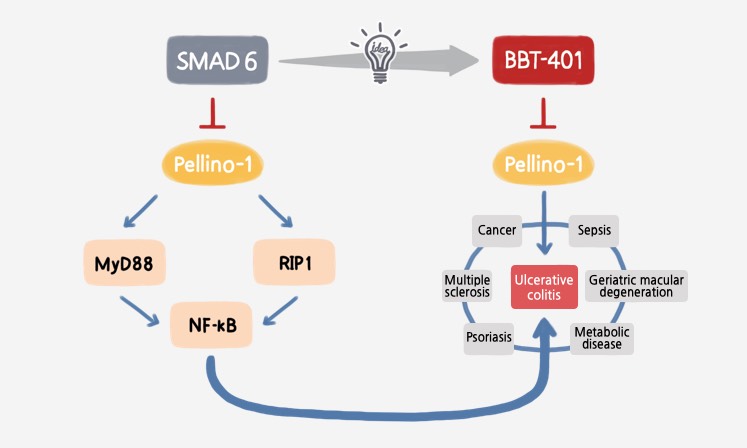
BBT-401
BBT-401 is the first Pellino-1 inhibitor, which demonstrated to be highly selective and potent at industry standard preclinical models. BBT-401 has the first-in-class and the best-in-disease potential for the treatment of ulcerative colitis.
| Indication / Disease more |
- Ulcerative colitis |
Target Protein |
- Pellino-1 |
| Development Stage |
- US IND approved / Phase 1 clinical trial in progress |
||
| Unmet Medical Needs |
- Poor patient compliance to first line therapies (5-ASA) - Side-effects of second line treatment options (Steroids, Immunosuppressants) - High price of third line treatment options (anti-TNF alpha and other biologics) |
Differentiation Strategy and Market Positioning |
- Novel target (Pellino-1) inhibitor - Confirmed excellent safety profiles Due to GI-tract restricted distribution - Confirmed superior efficacy with anti-inflammatory and intestinal mucosa healing |
| Intellectual Property Rights |
International patent applications and entries in 25 countries |
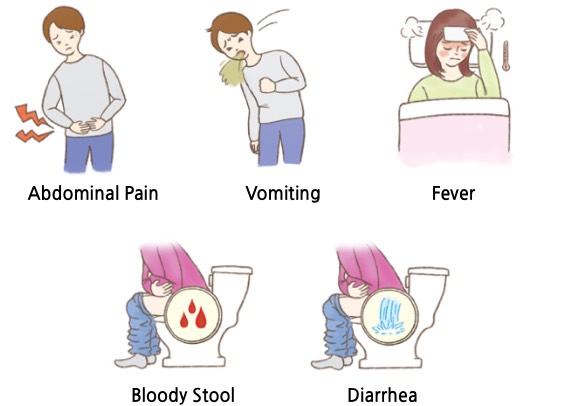
Ulcerative colitis is a type of inflammatory bowel disease (IBD) that causes inflammation or ulceration in the mucosa or submucosa of large intestine. Unlike Crohn’s Disease (inflammatory bowel disease sporadically occurring in the small intestine and large intestine) which is another type of IBD, UC is characterized by lesions that start at the rectum adjacent to the anus and connect to the inside of the colon. Most patients with ulcerative colitis have dilute stool or diarrhea with blood and mucus several times a day. Some severe cases involve abdominal pain, dehydration, fever, vomiting and weight loss. Symptoms other than large intestine may include nodular erythema, gangrene pneumonia, and oral ulcers, which may include ophthalmic arthritis and ankylosing spondylitis. Most cases of ulcerative colitis are chronic recurring. Symptoms disappear in few weeks after occurrence, and they repeat again over a period of months to years. The more the recurrence, the worse the condition becomes, and overall, 3-18% of the patients are known to develop colon cancer. In addition, acute fulminant colitis is very symptomatic and may require surgery to remove the entire colon within a few days without response to treatment. As first-line drug, 5-ASA (mesalamine) may be prescribed, where steroids may be prescribed depending on the symptoms. Biological agents including immunosuppressants may also be used. The market is divided into high efficacy/high-priced biologic agents and low efficacy/safe first-line drug. There has been a constant need for a new first-line drug which demonstrate excellent efficacy but with reasonable price.